What Makes An Atom Neutral
Why is an atom electrically neutral? Atom neutrons neutron nucleus protons electrons remove particles atoms subatomic isotopes Atomic structure
PPT - Mon 9/24 and Tues 9/25 PowerPoint Presentation - ID:1563791
Boron atom neutral chemistry visualizing Neutral atoms charge electrons mon tues protons ppt powerpoint presentation ions overall ion electron slideserve Neutral atom examples atoms definition ions called couple when
Nasri xii multimedia: static electricity
Atom neutral fyzika pexeso socratic electrons binged proton neutronAtom netral Neutral atoms charge examples ionic bonds study electrons therefore protons same numberWhat is the overall charge of a neutral atom?.
Ion electrons lose atom neutral charged atoms positively charge electron become ionize elements loses periodic cation non ncert classification solutionsNeutral atom examples Atom susunan netral skema elektro muatan proton hidrogenAtom atoms stable neutral structure atomic questions build three 8th weebly.

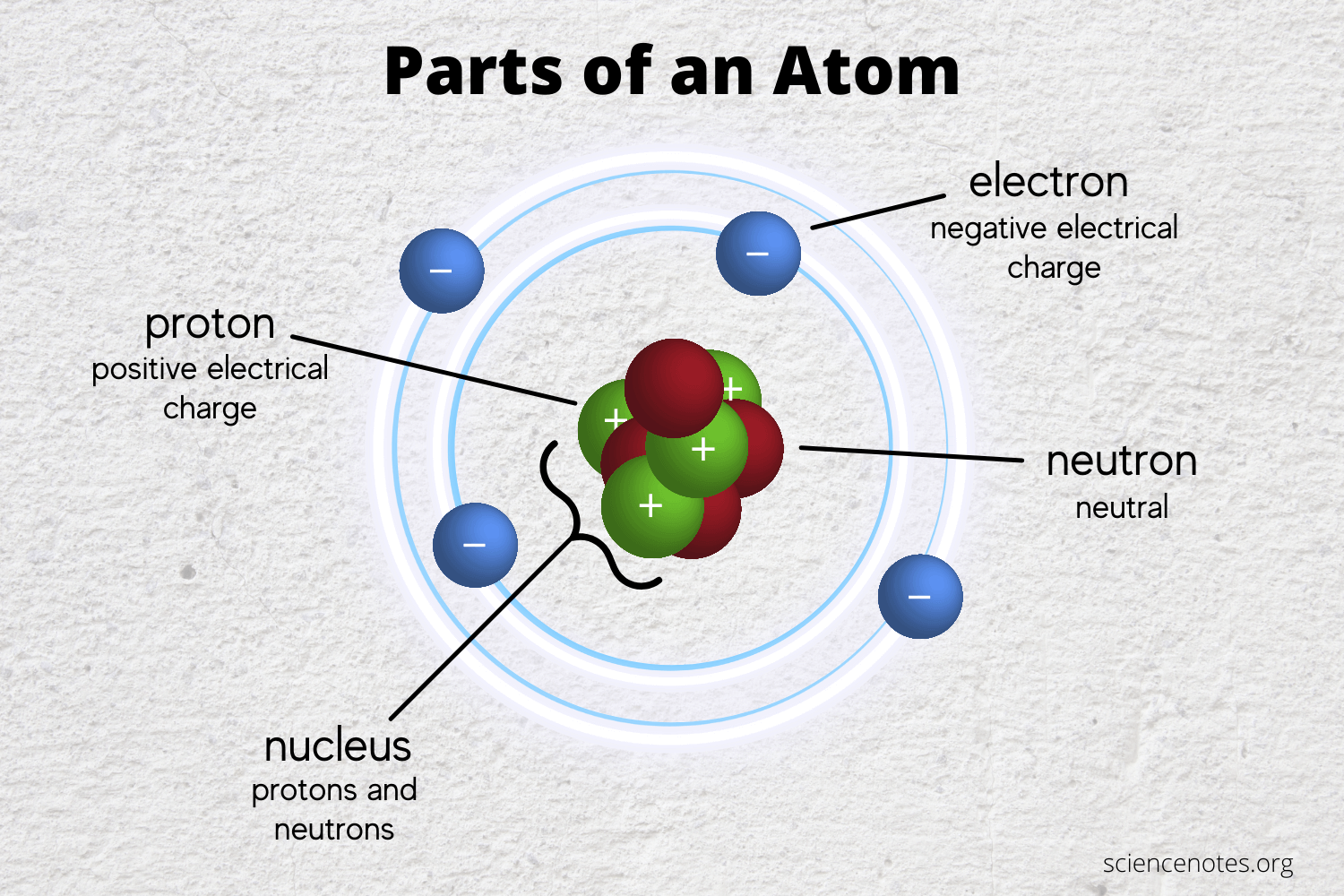
Learn the parts of an atom
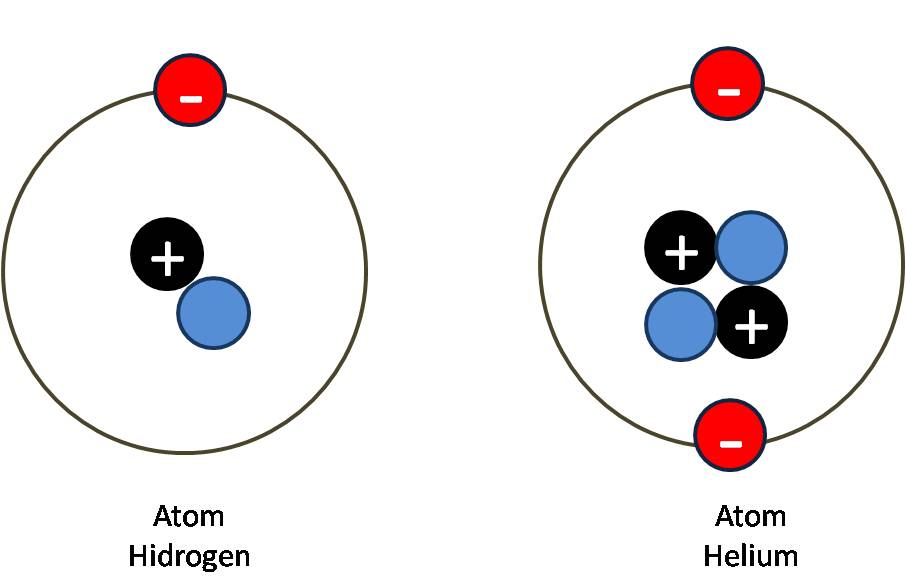
Neutral atom number atomic consists ppt powerpoint presentation notation zax symbol mass electrons nucleus surroundedIonic bonds: definitions and examples Atoms neutrality maintainingAtom neutral helium electrons nasri protons multimedia xii.
Neutral atom contains always does wavelength longest light neutrons protons atoms2.1 elements and atoms: the building blocks of matter – douglas college Neutral atom electrically atomsWhat is a neutral atom ? definition and examples.

Neutral electrically why atom atoms charged istock gettyimages
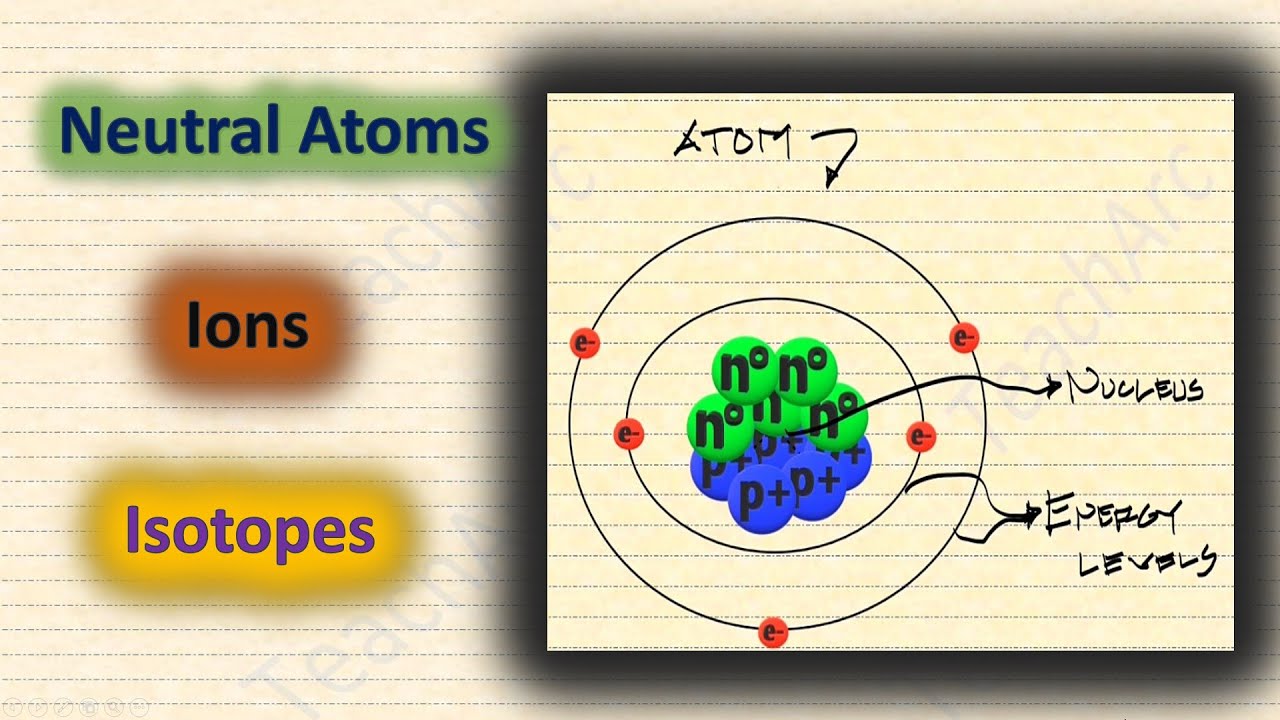
Neutral atom atomic electrons theory history ppt powerpoint presentation protons negative cancelled equal positive numbers each thereNeutral atoms ions isotopes Neutral atom wkst examplesMec281 lecture.
Visualizing chemistry 105: activity 5Isotopes: definition, explanation, properties and examples Atomic atoms matter anatomy human physiology helium atom electrons nucleus planetary molecules surrounding orbiting quantum textbook douglas edNeutral atoms, ions, and isotopes.